Platynothrus punctatus
Platynothrus punctatus has a Holarctic, boreo-alpine distribution, which means that it occurs in the northern latitudes, e.g., in nothern Norway, and at higher altitudes, e.g., at Hardangervidda in Norway, and Sierra Nevada in southern Spain. It prefers aquatic or damp microhabitats. Its reproduction is parthenogenetic, that is, only females are present and they are produced from unfertilized eggs.
- Innhold
- Description
- Look-alikes
- Biology
- Ecology
Description
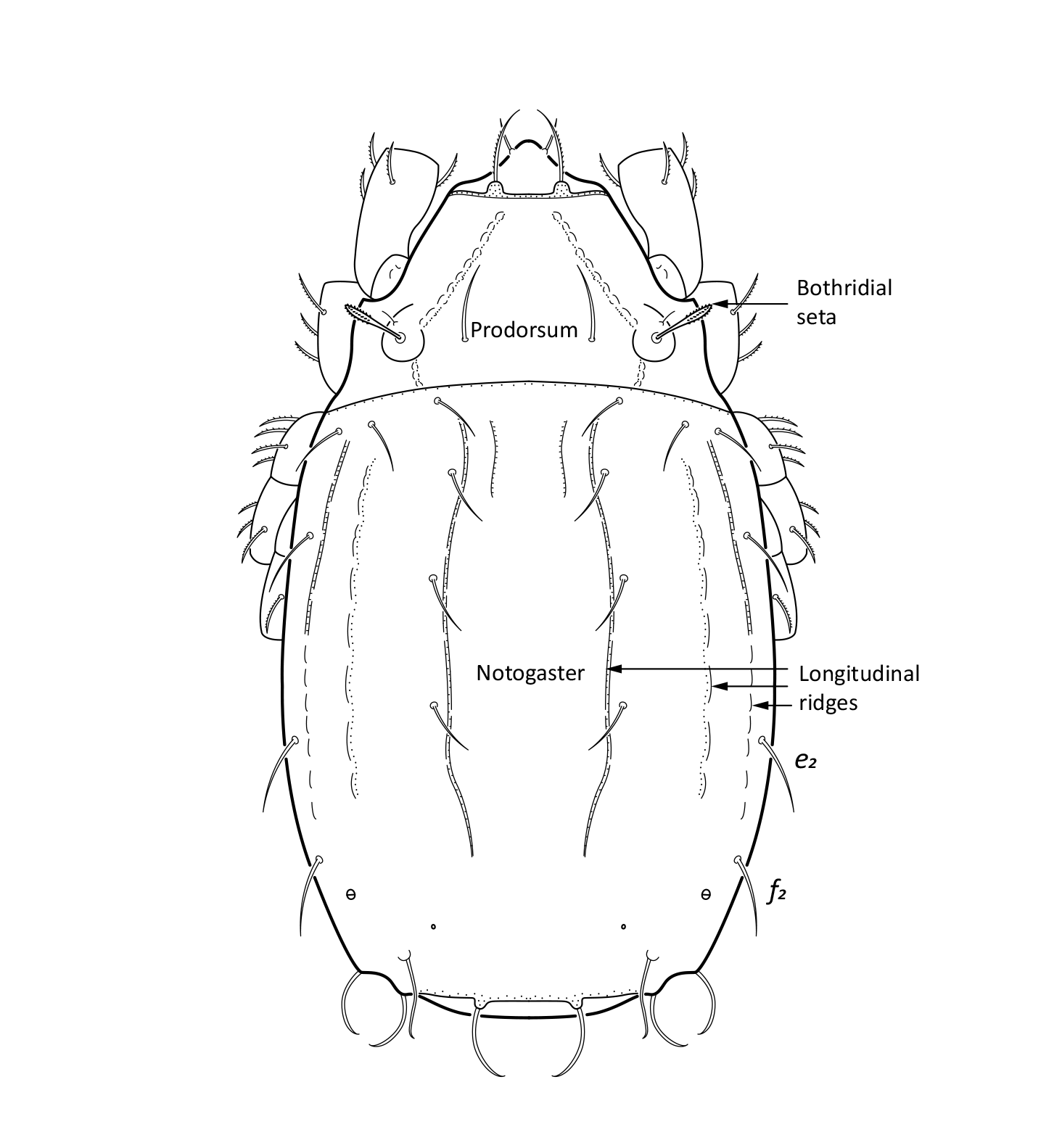
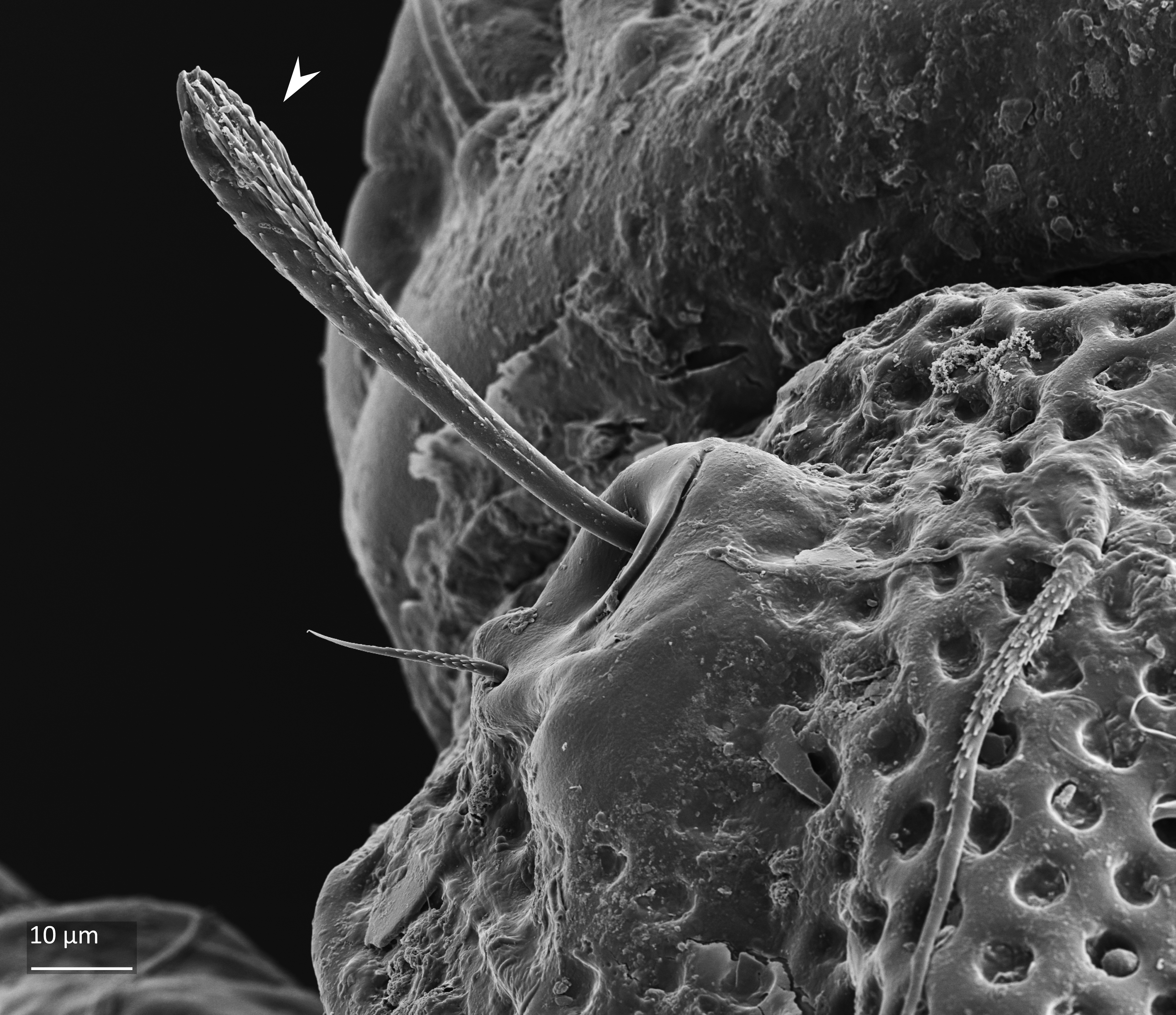
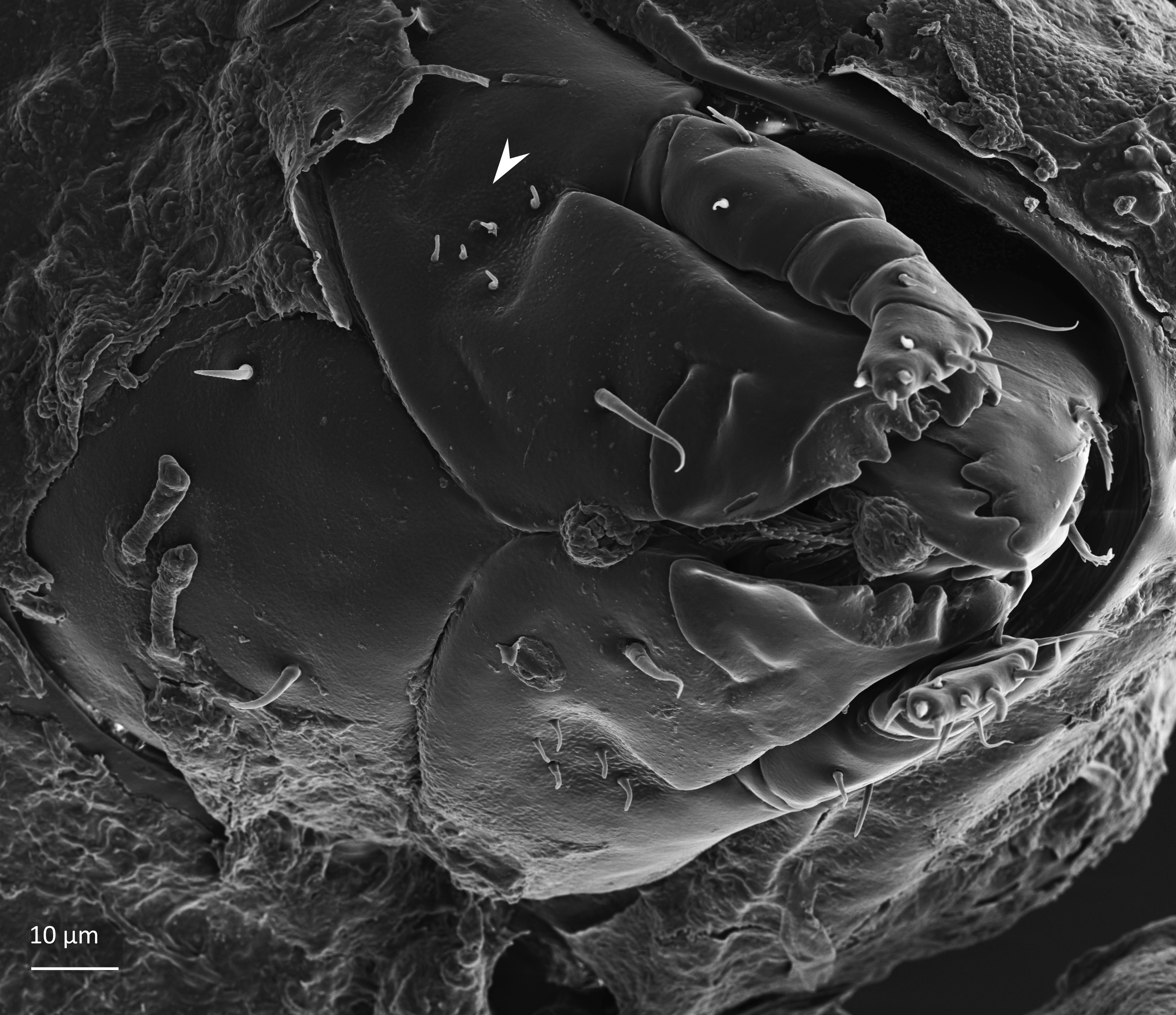
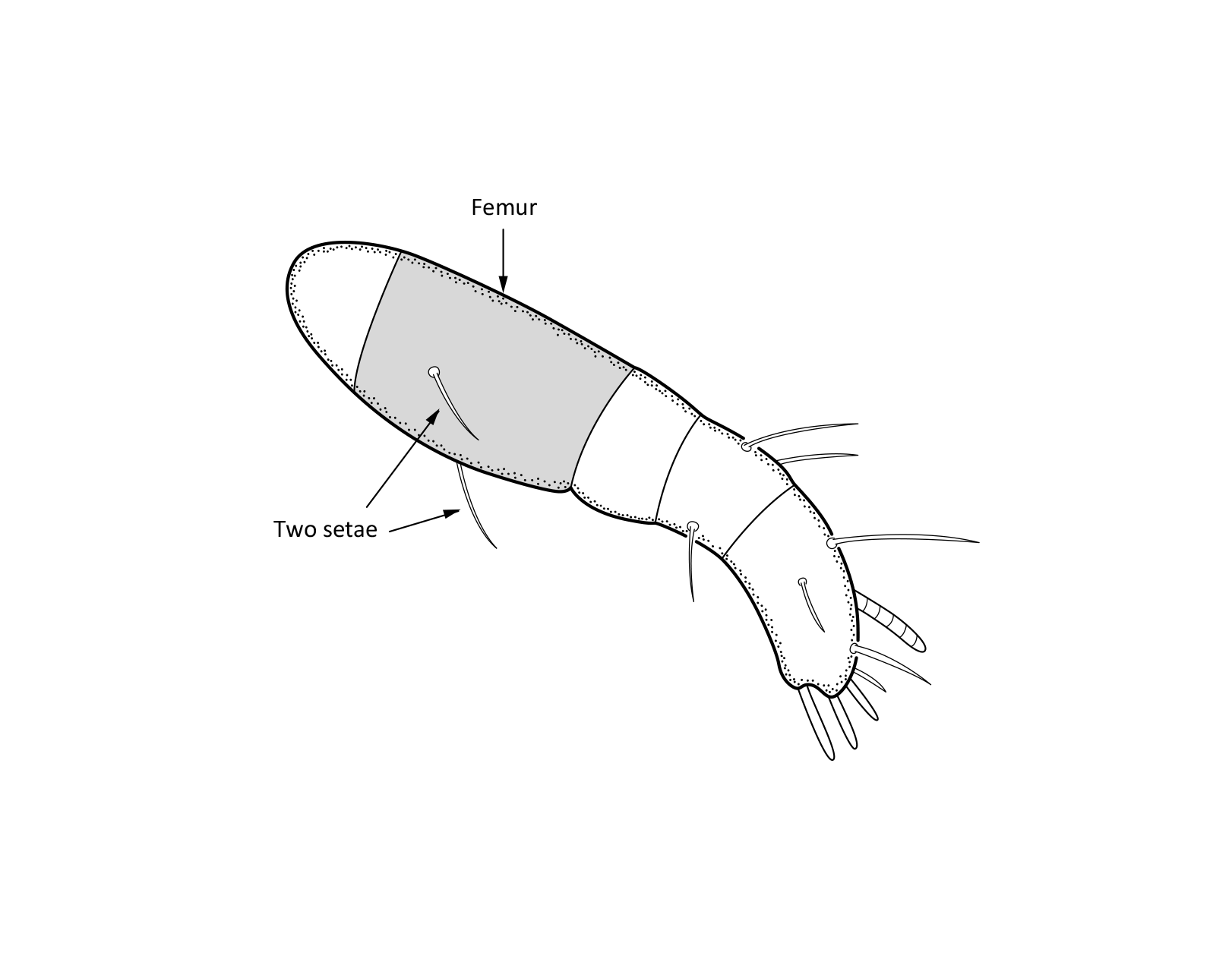
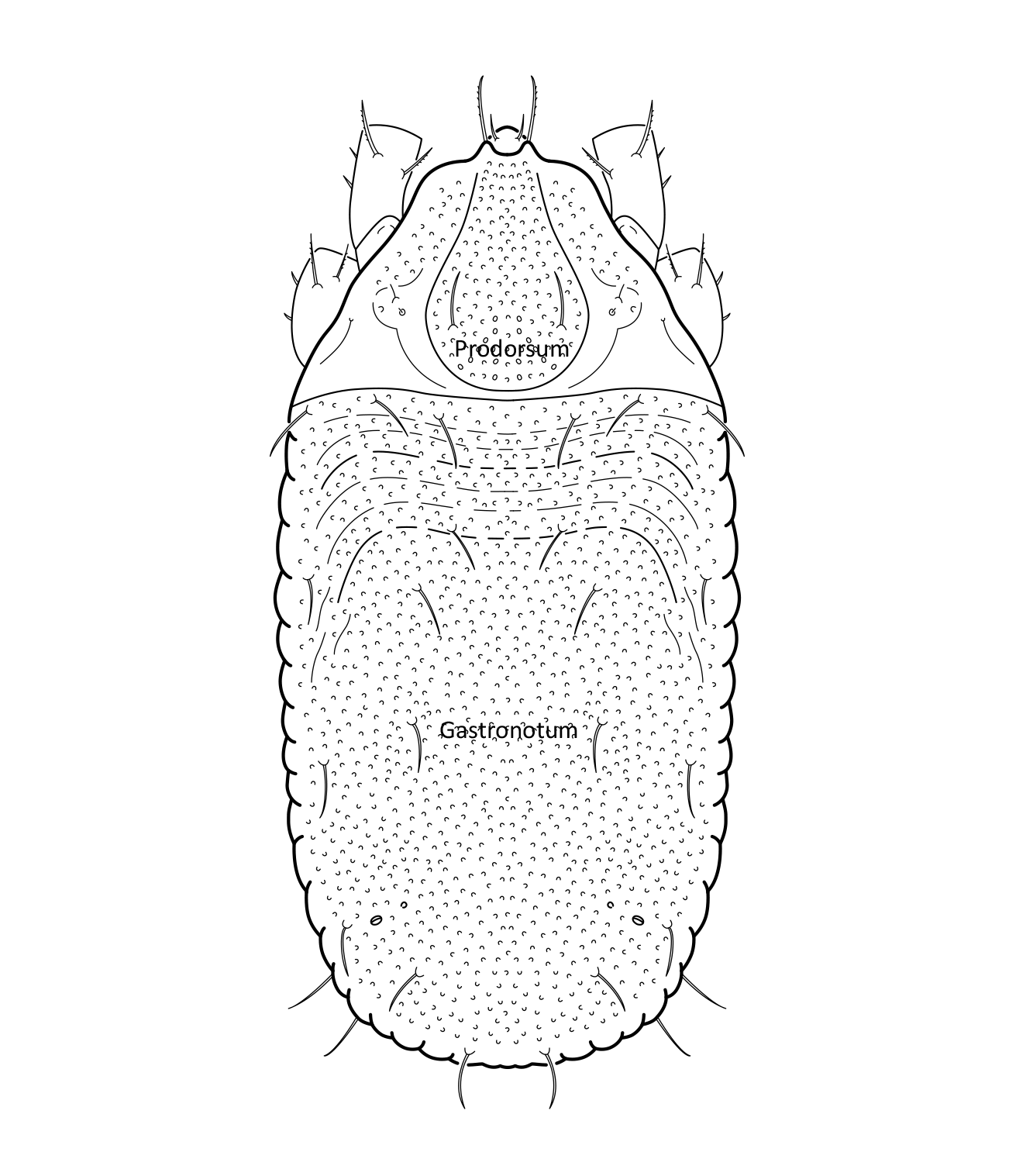
Adult: The length of the body is 734–864 µm, and the width is 408–505 µm. The color is dark brown, almost black. The bothridial seta is medium sized, bacilliform, with slightly thickened and barbed head (Figs. 1 and 2). The notogaster has three pairs of longitudinal ridges and 15 pairs of medium sized setae; the notogastral seta f2 is as long as seta e2 (Fig. 1). The adult has five pairs of hyposthomal setae m (Fig. 3) and a palp femur with two pairs of setae (Fig. 4). Three pairs of adanal setae and two pairs of anal setae are present. The leg tarsi have one claw (Seniczak et al. 2022).
Juvenile stages: Larva: body length 284 µm, width 125 µm. Protonymph: body length 351 µm, width 155 µm. Deutonymph: body length 416 µm, width 172 µm. Tritonymph: body length 572 µm, width 305 µm. The color is whitish, however, the prodorsum, the epimeres and the legs are light brown. The prodorsum is subtriangular, with the central part punctate and with small pits (Figs. 5 and 6). The palp femur has two pairs of setae, like in the adult. The number of hyposthomal setae m varies in juvenile stages: the larva has 1 pair of these setae, the protonymph has 2 pairs, the deutonymph 3 pairs, and the tritonymph has 4 pairs (Seniczak et al. 2022).
Fig. 1. Dorsal view of Platynothrus punctatus, adult; notogastral setae f2 and e2 are marked in the figure.
Fig. 2. Frontal view of adult Platynothrus punctatus; arrowhead points slightly thickened and barbed head of bothridial seta.
Fig. 3. Ventral view of gnathosoma of adult Platynothrus punctatus; arrowhead points five pairs of hypostomal setae m.
Fig. 4. Palp of adult Platynothrus punctatus.
Fig. 5. Dorsal view of Platynothrus punctatus, juvenile (tritonymph).
Look-alikes
Platynothrus punctatus is similar to Platynothrus troendelagicus A. et S. Seniczak 2022, but its adult has slightly thickened bothridial seta while Platynothrus troendelagicus has a clearly thickened bothridial seta. Platynothrus punctatus has notogastral seta f2 as long as e2 while in Platynothrus troendelagicus the seta f2 is longer than e2. In Platynothrus punctatus the number of hyposthomal setae m varies in the developmental stages: the larva has 1 pair, the protonymph has 2 pairs, the deutonymph has 3 pairs, the tritonymph has 4 pairs, and the adult has 4 pairs. In Platynothrus troendelagicus all developmental stages have 1 pair of hyposthomal setae m.
Biology
The reproduction is parthenogenetic (Maraun et al. 2019), that is, only females are present, and they are produced from unfertilized eggs.
Fig. 6. Dorsal view of Platynothrus punctatus, juvenile (tritonymph).
Ecology
Distribution
Platynothrus punctatus has a Holarctic, boreo-alpine distribution (Subías 2004, 2023). It is very common in the Arctic region (Majka et al. 2007, Coulson et al. 2015), and in alpine habitats (Trägårdh 1910, Solhøy 1975, Hågvar et al. 2009), from northern Europe (Sweden, Norway) to southern Spain (Seniczak et al. 2022).
Habitat
The species prefers aquatic or damp microhabitats such as mosses in slow-flowing water, springs and brooks, and mosses on the shore of lakes and ponds, including brackish ponds (Majka et al. 2007), but has also been recorded from less humid habitats, for example from wet meadows with thick moss (Solhøy 1975, Seniczak et al. 2022). It is common in arctic soils Behan-Pelletier and Lindo 2023). It has also been found in the feathers of birds (Lebedeva et al. 2006).
Findings in Norway
Platynothrus punctatus is common in Norway in moist alpine habiats and at northern latitudes (Northern Norway, Svalbard, Jan Mayen) Thor 1937, Karppinen 1971, Solhøy 1975, Seniczak et al. 2006, 2010, Coulson et al. 2015). At a glacier foreland in Finse this species was absent at the youngest moraine (deglaciated 32–48 y ago) and started to appear at the moraines deglaciated 52–66 y ago. It had similar abundance (about 2,000 indiv. Per m2) and a similar dominance (about 5.5%) in the oribatid communities in the entire gradient towards the oldest moraine (deglaciated ca. 10,000 y ago) (Hågvar et al. 2009).
References
Behan-Pelletier VM and Lindo Z (2023). Oribatid Mites. Biodiversity, Taxonomy and Ecology. CRC Press, 508 pp.
Coulson SJ, Fjellberg A, Melekhina EN, Taskaeva AA, Lebedeva NV, Belkina OA, Seniczak S, Seniczak A and Gwiazdowicz DJ (2015). Microarthropod communities of industrially disturbed or imported soils in the High Arctic; the abandoned coal mining town of Pyramiden, Svalbard. Biodiversity and Conservation 24(7), 1671–1690. doi.org/10.1007/s10531-015-0885-9
Hågvar S, Solhøy T and Mong CE (2009). Primary succession of soil mites (Acari) in a Norwegian glacier foreland, with emphasis on oribatid species. Arctic, Antarctic, and Alpine Research 41(2), 219–227. doi.org/10.1657/1938-4246-41.2.219. Studies on the Oribatei (Acari) of Norway. Annales Entomologici Fennici 37, 30–53.
Lebedeva NV, Lebedev VD and Melekhina EN (2006). New data on the oribatid mite (Oribatei) fauna of Svalbard. Doklady Biological Sciences 407, 182–186.
Majka CG, Behan-Pelletier V, Bajerlein D, Bloszyk J, Krantz GW, Lucas Z, OConnor B and Smith IM (2007). New records of mites (Arachnida: Acari) from Sable Island, Nova Scotia, Canada. The Canadian Entomologist 139(5), 690–699. doi.org/10.4039/n06-103
Maraun M, Caruso T, Hense J, Lehmitz L, Mumladze L, Murvanidze M, Nae I, Schulz J, Seniczak A and Scheu S (2019). Parthenogenetic vs. sexual reproduction in oribatid mite communities. Ecology and Evolution 9(12), 7324–7332. doi.org/10.1002/ece3.5303
Seniczak A, Solhøy T and Seniczak S (2006). Oribatid mites (Acari: Oribatida) in the glacier foreland at Hardangerjøkulen (Norway). Biological Letters 43(2), 231–235.
Seniczak A, Solhøy T, Seniczak S and Riva-Caballero A (2010). Species composition and abundance of the oribatid fauna (Acari, Oribatida) at two lakes in the Fløyen area, Bergen, Norway. Biological Letters 47(1), 11–19. doi.org/10.2478/v10120-009-0014-0
Seniczak S, Seniczak A, Kaczmarek S, Marquardt T, Ondoño EF, Coulson SJ (2022). Morphological ontogeny and ecology of Platynothrus punctatus (Acari, Oribatida, Camisiidae), with comments on Platynothrus Berlese. Systematic and Applied Acarology 27(3), 551–580. doi.org//10.11158/saa.27.3.12
Solhøy T (1975). Dynamics of Oribatei populations on Hardangervidda. In: FE Wielgolaski (ed.), Fennoscandian Tundra Ecosystems, Part 2. Ecological Studies. Analysis and Synthesis. Berlin-Heidelberg-New York, Springer, pp. 60−65.
Subías LS (2004, updated 2023). Listado sistemático, sinonímico y biogeográfico de los Ácaros Oribátidos (Acariformes, Oribatida) del mundo (1758−2002). Graellsia 2004, 60 (número extraordinario), 3−305. 18 actualization, 540 pp.
Thor S (1937). Übersicht der norwegischen Cryptostigmata mit einzelnen Nebenbemerkungen. Nyt Magazin for Naturvidenskaberne 77(1), 275–307.Trägårdh I (1910). Acariden aus dem Sarekgebirge. Naturwissenschaftliche Untersuchungen des Sarekgebirges in Schwedisen-Lappland, Zoology, Stockholm 4, 375−586.